May 18, 2017 - by Simone Ulmer
Hydrogen is a promising environmentally friendly source of energy, which is why researchers have long dreamed of using sunlight to provide sustainable and virtually inexhaustible energy through the production of hydrogen from water. Their inspiration is the formation of oxygen by photosynthesis in green plants and algae, especially those processes that take place in what scientists call photosystem II. Photosystem II plays a role in the first half of the photosynthesis reaction, where water splitting occurs. Many details of this process are yet to be understood.
Sandra Luber, Professor of Theoretical Chemistry at the University of Zurich, takes the natural photosynthetic process as the basis for her research on catalysts for water splitting. She is particularly interested in the oxidation of water to form oxygen, which provides electrons and protons essential for hydrogen production. “Water oxidation is still a limiting crucial step to split water artificially,” says Luber. This is due to a lack of robust and efficient water oxidation catalysts.
Mimicking natural catalysts
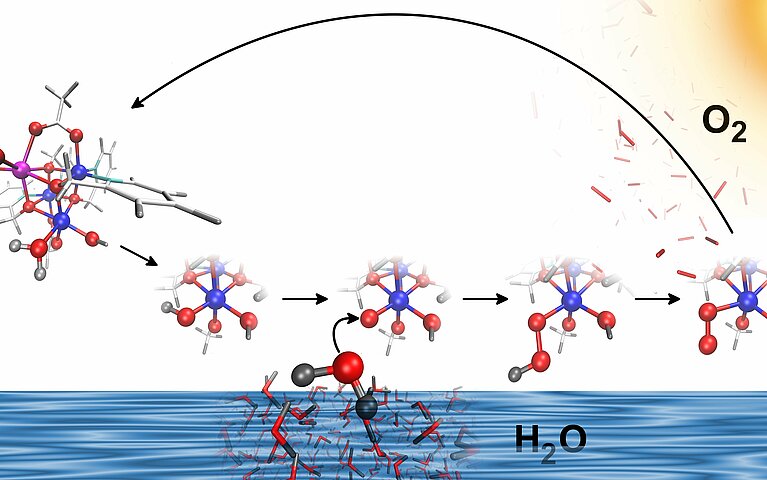
In natural photosynthesis, the water oxidation catalysts in photosystem II have a cube-shaped structure. The cube is built from manganese atoms and a calcium atom linked together via oxygen atoms. Although the structure and composition of this cubic catalyst is now well researched, to date there has been no success in developing a structurally comparable, functioning artificial catalyst in the laboratory. Luber says that an artificial cubic catalyst was able to perform water splitting at all only by using cobalt instead of manganese for the metal atoms in the structure. Luber and her team are using the CSCS supercomputer ‘Piz Daint’ to investigate the secrets of this cube structure that positively influence catalytic activity and the role played by the flexibility of its shape, in efforts towards one day building the ideal catalyst.
In her models, Luber inserted a lanthanide into the cube together with the cobalt. In the natural photosystem II, the cube instead uses the element calcium in its place. The calcium and lanthanide respectively stay unchanged during the reaction and maintain a constant oxidation state, whereas the cobalt atoms give away or take up electrons and thus are constantly changing their oxidation states.
Analogous to the calcium in photosystem II, the lanthanide further increases the activity of the catalyst, explains Luber. This has been observed experimentally by the group of Professor Greta Patzke at the University of Zurich, as well as in Luber’s calculations. But why it happens had not been entirely clear. Luber and her team developed a model to specifically investigate this effect. It became evident that the acetates (salts and esters of acetic acid) bound to the metal atoms are highly flexible. “The acetates are indeed so flexible that they easily exchange with water or an OH group,” Luber points out. Splitting water calls for a steady feed of fresh water, and such an exchange conveys it more easily to the active centre where the splitting reaction occurs.
The researchers also observed that groups bound to the lanthanide can act as a base: the lanthanide OH groups can accept protons from the water and thereby lower the reaction barrier. “That makes the process run faster”, explains Luber.
The researchers also discovered that a cube built from cobalt and a lanthanide is structurally flexible: the cobalt can easily be pulled out of the cube. This is an effect already observed in nature, but now for the first time it has been shown to occurr in an artificial catalyst. Luber suspects that this flexible behaviour further accelerates the catalytic mechanism.
Catalyst behaviour rendered visible by molecular dynamic calculations
The researchers discovered the catalyst’s flexing behaviour by chance. Luber explains that this was a consequence of the fact that the calculations performed on ‘Piz Daint’ were not based on the usual standard static approach. Instead, the researchers used the CP2K code to perform ab initio (density functional theory, or DFT) and molecular dynamics calculations, taking into account the quantum states of the electrons in an atom.
Luber does not venture to predict how long it will be before hydrogen can be used straightforwardly as a fuel. Many theoretically promising catalysts for water splitting have been discovered which have proved to be unstable in nature. This is why Luber collaborates with Patzke: Patzke and her research group are pursuing by experimental means the same goal: using artificial photosynthesis to produce hydrogen as an attractive alternative energy source to fossil fuels. A total of nine research groups at the University of Zurich are at work on the Solar Light to Chemical Energy Conversion (LightChEC) project, where the aim is to develop a highly efficient system for artificial water splitting and sustainable hydrogen production.
Dream of a theory breakthrough
This year Luber became the first theoretician to receive the Clara Immerwahr Award, and she also holds one of just two professorships in chemistry funded by the SNSF. “A funded professorship in theoretical chemistry is rather unusual,” says Luber. She finds that all the more pleasing because it gives recognition to the significance of computer-aided chemistry. And the professor emphasises: “My theoretical research is not possible without high-performance computers like ‘Piz Daint’.” Catalysis is only one of Luber’s research areas. Her dream is one day to come up with a tool or theoretical approachthat could allow the specific development and identification of materials with particular desired properties. So far, theory and practice have both tended to use expensive trial-and-error methods. But if Luber manages to turn the tables, that would be groundbreaking for materials research.
Further reading
- Hodel FH & Luber S: Redox-Inert Cations Enhancing Water Oxidation Activity: The Crucial Role of Flexibility, ACS Catal. (2016), 6, pp 6750–6761, DOI: 10.1021/acscatal.6b01218
- Hodel FH & Luber S: What Influences the Water Oxidation Activity of a Bioinspired Molecular CoII4O4 Cubane? An In-Depth Exploration of Catalytic Pathways, ACS Catal. (2016), 6, pp 1505–1517, DOI: 10.1021/acscatal.5b02507