March 09, 2020 - by Santina Russo
In recent years, perovskites speedily emerged as promising new materials for future solar cells. Although they have been investigated only for a comparatively short time, solar cells made out of certain perovskite materials already exceed 22% efficiency in converting solar light to electrical energy under lab conditions, which is more than commercial silicon cells. However, not all perovskite materials exhibit such favourable properties, and the physics behind their photovoltaic performance is not yet fully known.
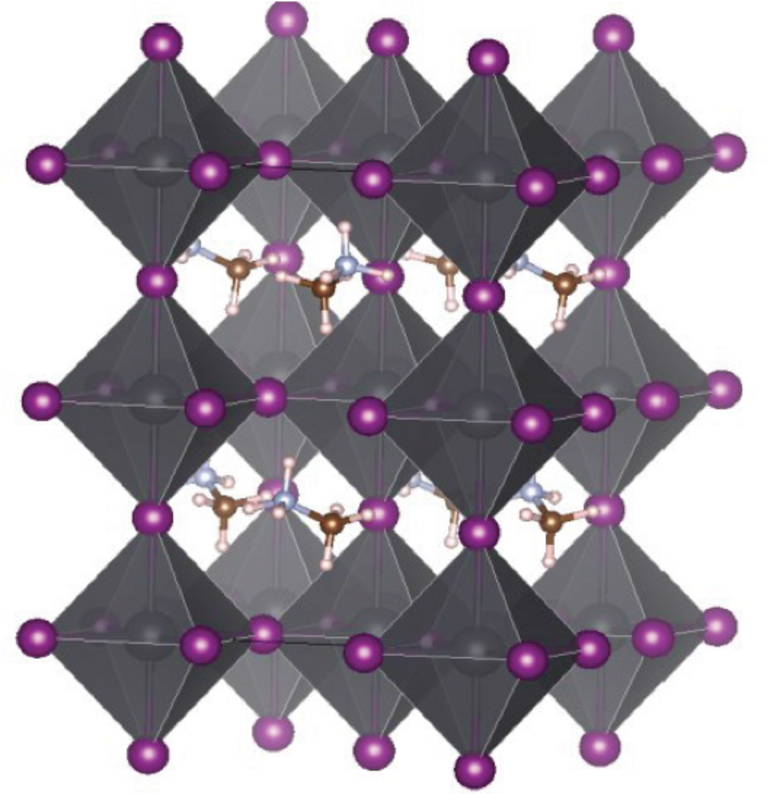
“Understanding these processes is important, since this will facilitate the design of new materials with favourable properties in the future”, says Alfredo Pasquarello, a professor in theoretical condensed matter physics at the EPF Lausanne. That is why he and his colleagues at EPF Lausanne used the “Piz Daint” supercomputer at CSCS to investigate a perovskite material called methylammonium triiodoplumbate (CH3NH3PbI3) — a material that can harvest sunlight with excellent efficiency because of a vital property: the exceptionally long lifetime of its charge carriers.
The importance of the charge transfer rate
In photovoltaic cells, the energy from the sunlight excites electrons, bringing them from their usual state to the higher-energy conduction band. There, the electrons can move freely in the material and become charge carriers. Their movement creates an electrical current, that can be relayed and harvested. Likewise, an electron hole, which is the positive charge occurring from the lack of an electron where one could exist, can also travel through the material acting as a charge carrier.
This means that for a material to work well for solar cells, its electrons must be easily excitable. But, this is only one part of the requirement: “More important is actually a fast and efficient charge transfer within the material”, explains Pasquarello, and this property is mostly determined by the lifetime of the charge carriers — the electrons and electron holes. However, in many materials the charge carrier lifetime is limited, because the electrons quickly fall back from their excited state. They re-integrate themselves into the electron-holes and adopt a fixed position, thereby ending the charge transfer — a process that is called electron-hole recombination.
The rate of this recombination determines how efficiently the material can maintain and transport the harvested energy. For instance, the well-known perovskite CH3NH3PbI3 is stable in different crystal structures, including a tetragonal structure and an orthorhombic structure. One of those, the orthorhombic structure, shows a high electron-hole recombination rate, and therefore a correspondingly low charge carrier lifetime. As a result, it was found to have extremely poor charge transfer properties. In contrast, the tetragonal structure exhibits a far lower recombination rate and therefore much better — in fact outstanding — charge transfer capabilities.
Explaining an exceptionally long lifetime
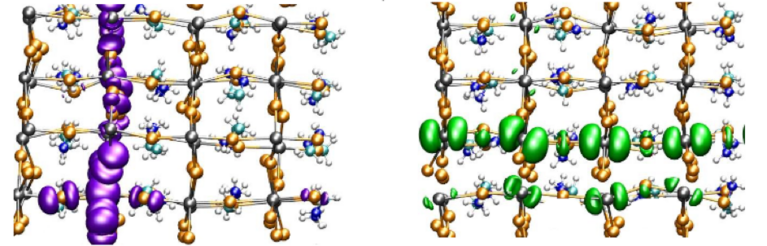
But what is the origin of these substantial functional differences? To solve this riddle, Alfredo Pasquarello and his team employed “Piz Daint” to simulate the excitation of electrons in the tetragonal CH3NH3PbI3 material by ab initio molecular dynamic simulations. The scientists either introduced an extra electron, an extra hole, or an electron-hole pair into the system and simulated the behaviour of these charge carriers to reveal the origin of their long-lasting lifetimes.
The research team found that, actually, subtle structural disorders within the material are responsible for the favourable lifetimes of the charge carriers. These minuscule disorders arise from polar effects within the crystal structure of the material. Specifically, thermal distortions and vibrations in the sublattice formed by the PbI3- ions lead to a consistent spatial separation of electrons and electron-holes. And this spatial separation proved to be key: It prevents electron-hole recombination, leading to the long charge carrier lifetime. “Achieving such a distinctly separate localization of electrons and holes is therefore fundamental for the performance of a photovoltaic material“, concludes Pasquarello — a finding, that should help to design new efficient materials for future solar cells.
(Image on top: Greg Stewart / SLAC National Accelerator Laboratory)
References:
- Ambrosio et al.: Origin of low electron–hole recombination rate in metal halide perovskites (2018) https://pubs.rsc.org/en/content/articlelanding/2018/ee/c7ee01981e#!divAbstract
- Bischoff et al.: Nonempirical hybrid functionals for band gaps of inorganic metal-halide perovskites (2019) https://journals.aps.org/prmaterials/abstract/10.1103/PhysRevMaterials.3.123802