January 15, 2020 - by Santina Russo
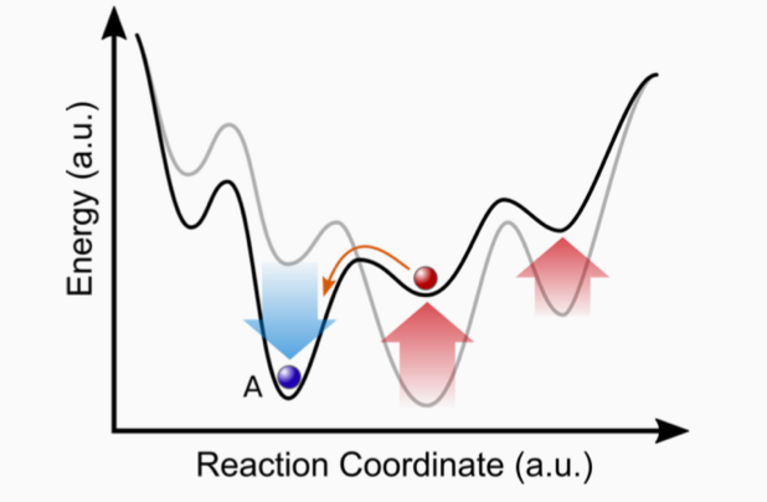
“Diamonds are forever”, Shirley Bassey sang in the 1980s, underlining that the famous gems are in fact made of the hardest naturally occurring material on Earth. But diamonds are also a thermodynamic peculiarity: They are only metastable compared to the graphite form of carbon, meaning that it is not their energetically most favourable state and the right conditions must be maintained for them to form. That’s why industrial diamonds are produced under extreme conditions of temperature and pressure, similar to the conditions needed for their natural formation. Once formed at such extreme conditions, a material can get trapped in a local energy minimum and the metastable state can retain its stability even in normal environmental conditions, but only if the kinetic barriers of the local minimum are just high enough — as is the case with diamonds.
Similarly, some promising new materials can be synthesized at very high temperature or pressure — ideally in such a way that they exhibit improved properties to both enhance present applications and to better address global ecological and economical challenges. Two active fields of recent high-pressure materials discoveries are high-temperature superconductors and materials possessing outstanding light absorption properties or charge transfer rates, thereby yielding more efficient solar cells. A few years ago, scientists at the Geophysical Laboratory of the Carnegie Institution in Washington managed to create a novel metastable material from silicon and sodium under high pressure that demonstrates better light absorption than current silicon solar cells. And just recently, a new phase of lanthanum-hydride was synthesized under high pressure that exhibits the highest superconducting transition temperature observed to date. The basis for this experimental work was utilizing extensive computer simulations to determine the precise chemical composition and structure, and the conditions needed to form the new material. “Since high-pressure experiments in the laboratory are very difficult and elaborate, previous simulations are crucial”, says Maximilian Amsler, Postoc in the group of Ulrich Aschauer at the University of Bern and at Cornell University.
A smart combination
As essential as computer simulations are, they are limited as well: existing methods were computationally expensive and not suitable for simultaneously exploring chemical compositions and structures at high pressures on a large scale. The physicist Amsler and his former team at Northwestern University, USA, used the Piz Diant supercomputer at CSCS to develop a computational framework that allows researchers to assess non-ambient conditions much more efficiently — and with this, explore the whole space of possible high-pressure materials.
To achieve this, the researchers extended and combined two existing methods. On the one hand, there are data-driven approaches that rely on large databases of computed properties of known materials. These methods can be used to analyse the thermodynamic stability of hundreds of thousands of chemical compounds. However, it is mostly limited to idealized conditions — actually zero temperature and pressure. On the other hand, methods to explore structures that emerge at high pressures have also been established, but they require computational resources that are too extensive to be practical for systematic combinatorial searches over wide chemical spaces. Such structure prediction methods are therefore only used to investigate very specific, limited chemical systems.
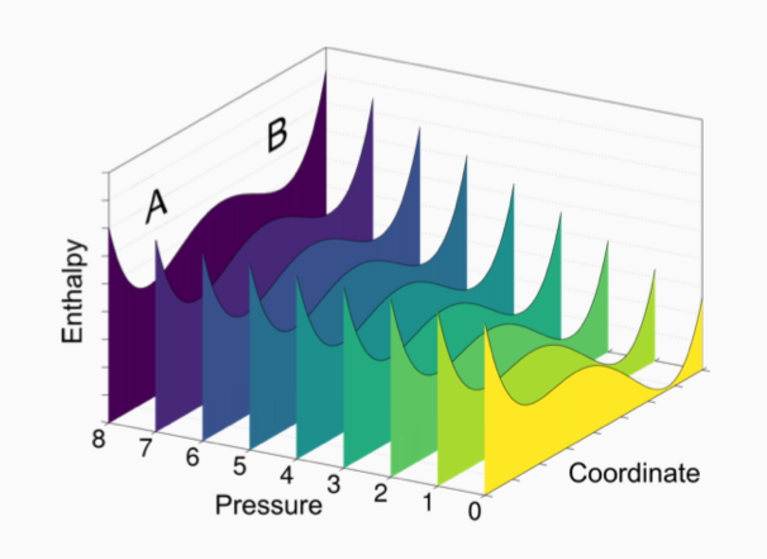
“The key was to sensibly combine those two approaches”, says Amsler. First, he developed a way to extrapolate high-pressure information from computational materials databases by employing a simple linear approximation to enthalpy, which is the value that determines a compound’s thermodynamic stability. This procedure was then used to extract promising compositions for high-pressure compounds from the Open Quantum Materials Database (OQMD). All compounds in the database — that is around half a million — were processed to predict new binary high-pressure materials, meaning compounds made from two different chemical elements. This approach requires far less computational resources but works well: of the compounds that had previously been shown to be stable at high-pressures in experiments, 80 percent were also predicted to be so by the new method.
Nine out of ten confirmed
In total, Amsler’s calculations with the OQMD yielded over 3,800 novel binary chemical compounds predicted to be stable at high pressures. In a second step, this information was used as guidance for the more computationally expensive structural searches. Of the most promising of the predicted compounds that could be readily realized with existing experimental techniques, 10 were selected for this validation process. The structural searches were carried out in parallel on Piz Daint, yielding a very positive result: of the 10 selected compounds, 9 were confirmed. “This demonstrates that we have found a rapid and robust way to explore the stability of chemical compounds at high pressures”, Amsler comments.
Apart from these reliable predictions, the calculations using the OQMD unexpectedly showed something else: out of the compounds that were previously known experimentally and that are only metastable at normal ambient conditions, up to 60 percent are actually thermodynamic ground states at higher pressures. “This suggests that high-pressure conditions should probably play a bigger role in future efforts of materials discovery”, Amsler concludes.
Reference:
Amsler M, Hegde VI, Jacobsen SD & Wolverton C: Exploring the High-Pressure Materials Genome, Phys. Rev. X 8, 041021 (2018), DOI: https://doi.org/10.1103/PhysRevX.8.041021