February 06, 2023 - by Simone Ulmer
Zeolites are porous minerals of the aluminosilicate group that are ubiquitous in our everyday lives. They got their name due to the fact that they give off water when heated and appear to boil (ancient Greek zéō) in the process. When they are heated, the water in the zeolites’ cavities evaporates, so they keep their original structure only up to water boiling temperature: With increasing temperatures, the water is lost, their pores (cavities) contract, and consequently their structure collapses.
Experimental researcher Georgia Cametti from the research group of Sergey Churakov, Professor and Head of the Mineralogy Department at the Geological Institute of the University of Bern, and colleagues have now made a surprising discovery in experiments with a subsection of zeolites in the so-called stellerite group (STI zeolites), which could be of great interest for industrial applications. The results of the study were recently published in the journal Scientific Reports.
The crystal structure of zeolites is formed by closely linked tetrahedral-shaped building blocks —aluminium and silicon atoms coordinated by four oxygen atoms. These oxygen-bridged tetrahedra form a skeleton with small to medium cavities containing mostly water and ions. Due to this structure and their special properties, zeolites are suitable, among other things, as catalysts in chemical processes or as molecular sieves and ion exchangers, to filter toxins from soil or water during environmental remediation. On the other hand, zeolites adsorb moisture very well and accelerate the drying process in dishwashers, for example, or in medicine for wound healing. They serve as cat litter and are even used as a food supplement. There are 93 recognised minerals belonging to the zeolites group and over 150 artificially produced zeolite-like materials.
Stable at high temperatures
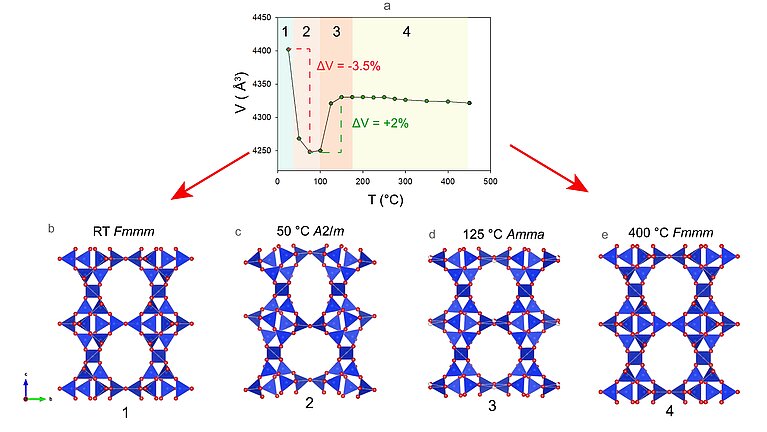
The STI zeolites now studied by the researchers at the University of Bern consist of a system of two-dimensionally interconnected channels. The cavities contain cations (positively charged ions), which can be easily exchanged with other ions. In their complex multi-method study, the research team used a natural zeolite with STI framework type whose original cations were replaced by lead (Pb) in the experiment—so-called lead-exchanged (Pb-exchanged) stellerites. When these Pb-zeolite forms were heated, the researchers observed that the crystals initially contracted continuously, as expected. However, it unexpectedly expanded again to its original volume with its original symmetry at a temperature of above 125°C.
Never observed behaviour
According to the researchers, such behaviour has never been observed before in zeolites. Sergey Churakov’s team has now found the explanation for the unusual behaviour through molecular dynamics simulations on CSCS’s “Piz Daint” supercomputer. "We first speculated that there are chemical reactions in the system, that basically the speciation of the cations is changing with temperature," says Churakov. "Speciation determines the chemical composition of lead complexes and thus their size. Normally one would expect that hydrated Pb2+ complexes will lose water molecules with higher temperature and become smaller in size, but there could be some dissociation of water and the oxidation of lead into Pb4+, which could build bigger complexes with OH- groups."
In order to get to the bottom of the cause for the unusual behaviour of the STI zeolites, the researchers tested four different scenarios in their simulations on “Piz Daint”. Using one of the scenarios, they could demonstrate that although the zeolites lose water as usual when heated, the hydroxyl groups made of hydrogen and oxygen are not lost but combine with the lead. As a result, large clusters of lead and hydroxides form in the cavities of the zeolites such that the framework structure is preserved and does not collapse. The pores, as well as the connections of the tetrahedra, remain intact, continuing to support the symmetry of the molecular crystal structure.
The researchers are convinced that the behaviour of the Pb-exchanged stellerites offers the possibility to use them in temperature ranges above 125°C. However, they admit that more studies are needed to determine whether this unusual trend applies to other Pb-exchanged zeolites. "Further research is aimed to reveal, if other transition metals with rich redox chemistry can extend the thermal stability of zeolites and thus broaden the range of their industrial applications," says Churakov. Here, large scale ab initio simulations play the critical role in understanding the molecular mechanism of corresponding structural transformations.
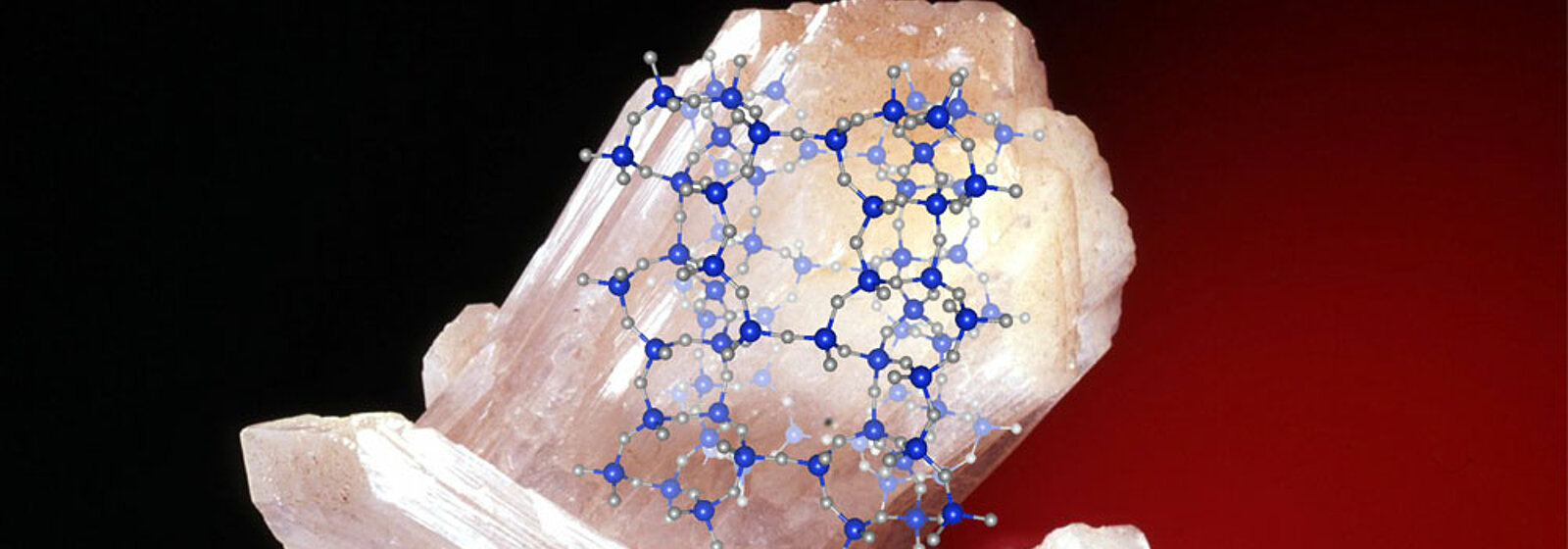
Image above: A natural zeolite crystal overlaid with its atomistic structure. (Image: University of Bern, Georgia Cametti & Sergey Churakov)
Literature reference & further information:
- Cametti G, Roos DP, Prieur D et al.: Pbx (OH)y cluster formation and anomalous thermal behaviour in STI framework-type zeolites. Sci Rep 12, 15934 (2022). https://doi.org/10.1038/s41598-022-20317-1
- Meet our User Sergey V. Churakov: https://www.cscs.ch/publications/stories/2022/meet-our-users-sergey-v-churakov/.
This article may be used on other media and online portals provided the copyright conditions are observed.