December 08, 2010 - by Simone Ulmer
Cells in living organisms continuously pull on the matrix fibrils surrounding them – on fibronectin protein fibres, for example. Consequently the fibronectin in an intact connective tissue environment is frequently stretched. If one cuts oneself, or enzymatic processes in an inflammation cause a kind of cut in the tissue, the tension on the fibronectin fibres is lost. Fibronectin dissolved in the blood then plays an important part in wound healing. It is woven into a network of fibres by the cells and initiates the first step in repairing the wound. The fibre tension does not build up again until the tissue repair process is started by fibroblasts – this is visibly signalled by the crinkly edges of the wound during healing.
Binding sites strung together like pearls
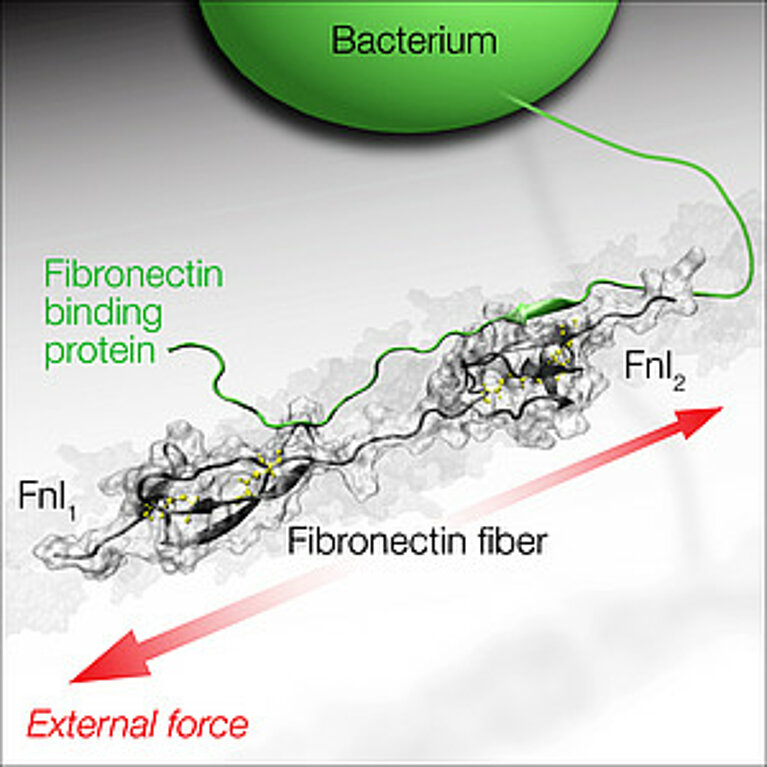
Fibronectin is structured like a necklace of about 50 “pearls”, and many of these “pearls” expose a binding site for molecules that are important in connective tissues, e.g. for collagens or cells. At the end of the molecule there are five “pearls” where bacteria can also bind to one of the “pearls” via certain segments of the long protein threads that decorate bacterial surfaces. Bacteria such as Staphylococcus aureus, the dreaded hospital germ and the typical pathogen of abscesses and boils, can specifically recognise fibronectin and thus infect wounds.
The research group led by Viola Vogel, Professor of Biologically Oriented Materials at ETH Zurich, has now studied how the tensile forces by which cells stretch connective tissue fibres affect the interaction between bacteria and fibronectin. The scientists did this by using a supercomputer to simulate how the structure of the complex between fibronectin and the bacterial protein molecule changes when a tensile force acts on fibronectin. Vogel’s doctoral student Samuel Hertig wanted to use this to find out whether the bacterial segments act like a kind of clamp during the docking process, thus preventing the fibronectin from stretching. However, the simulation shows an entirely different picture: although the bacterium initially binds to two sites on the fibronectin, it loses contact with the fibronectin molecule at one site as soon as a tensile force acts on the molecule, and the bond is destroyed. “The models give us a high-resolution picture of how the bond breaks. We would never see that using a microscope,” says Vogel. However, they also show that bacteria bind less well to stretched fibronectin fibres. The scientists conclude from this that the bacterial adhesion molecules which bacteria use to stick to tissue fibres are able to detect the physical state of the fibronectin molecule. Their study was published today in the e-journal “Nature Communications”.
Verified in the laboratory
Viola Vogel carries out research into the way mechanical factors modify the well-known classical equilibrium structures in biology, such as how stretching and pulling molecules changes the way they function. “However, since there are still no experimental tools with which we can determine structures that are not in equilibrium, we need to use supercomputers to calculate this,” says Vogel. Her team’s latest discovery is an impressive example of the success and usefulness of computer-aided sciences. The simulation enabled the researchers to deduce their theory, which could ultimately be verified by experiment.
They succeeded in this with the help of a method developed by the scientist and her team a few years ago, which makes the degree of stretching of the fibronectin visible through colours. However, since it is difficult to carry out binding studies in cell cultures, the researchers “built” a special experimental set-up in which they extracted fibronectin fibres and applied them to elastic substrates. Doctoral student Mamta Chabria was able to use this to show that the processes occurring with stretched fibronectin fibres in the presence of bacterial adhesion molecules are the same as those taking place in the model.
Since it is known that heparin, which is used in thrombosis prophylaxis, binds to fibronectin at the same sites as the bacteria, the scientists investigated whether large amounts of heparin provide protection against infections. “Although we could see that heparin does indeed reduce the binding of bacterial adhesion molecules to fibronectin, the bacteria respond to mechanical processes nonetheless,” says Vogel.
The knowledge that the tiny bacterium can recognise the physical state of a protein and that the stretching process changes the adhesion is essential to an understanding of biochemical processes. It involves one of the many interactions that are still undiscovered. Furthermore, only small elements of the entire adhesion region have been studied. “That is why we cannot say at present what long-term consequences the discovery will have – e.g. on the treatment of infectious diseases,” stresses Vogel. New computing time has already been requested at the Swiss National Supercomputing Centre CSCS in Manno, where the ETH Zurich professor carries out her modelling. She wants to resolve more questions, such as why different bacteria that bind to fibronectin have different numbers of adhesion molecules.
(Article with courtesy of ETH Life, ETH Zurich)
Reference
Chabria M, Hertig S, Smith LM & Vogel V: Stretching fibronectin fibres disrupts binding of bacterial adhesins by physically destroying an epitope, Nature Communications (2010). doi:10.1038/ncomms1135