November 12, 2012 - by Simone Ulmer
If the branched vascular system of a human were laid out in a line, the blood would have to cover approximately 100,000 kilometres to supply every last recess of the human organism with nutrients and oxygen. This impressive path, which cardiologists like to refer to, would effectively go round the world more than twice. Facing this is a delicate electrical conduction system in the heart, which keeps the blood circulation going: in a healthy person, the sinus node ensures that the heart beats at a rate of fifty to one hundred times a minute at rest. A intricate network of specialised cardiac muscle cells conducts every single electrical impulse to the ventricles, thus inducing them to contract. Through the contraction, the blood located in the ventricles is pumped into the circulatory system.
Detailed diagnosis not possible with ECG
For this to take place efficiently, the entire cardiac muscle has to contract at once. If the electrical conduction system is damaged, however, this is no longer guaranteed. One cause of an inefficient contraction of the heart can be a so-called left bundle branch block (LBBB). In this instance, the activation is only initiated in the right ventricle, which causes the left ventricle to contract in different places at different times. The blood is thus moved back and forth in the ventricle instead of being pumped out of it efficiently. This can lead to heart failure and the cardiac muscle can increase in size and expand. When this happens, cardiac resynchronization therapy using a biventricular pacemaker can be used in an attempt to improve the activation of the heart muscle.
“Resynchronization therapy breaks the vicious circle of deteriorating heart function and benefits 70 per cent of heart-failure patients with electrocardiogram signs of LBBB. But in the remaining 30 per cent it does not help, and can even impair the heart's performance,” says Mark Potse, a guest professor at the Università della Svizzera italiana. Although the electrocardiogram (ECG) points to an LBBB, the cause seems to lie partly or completely elsewhere in these patients. However, it is hard to make a detailed diagnosis with a conventional ECG.
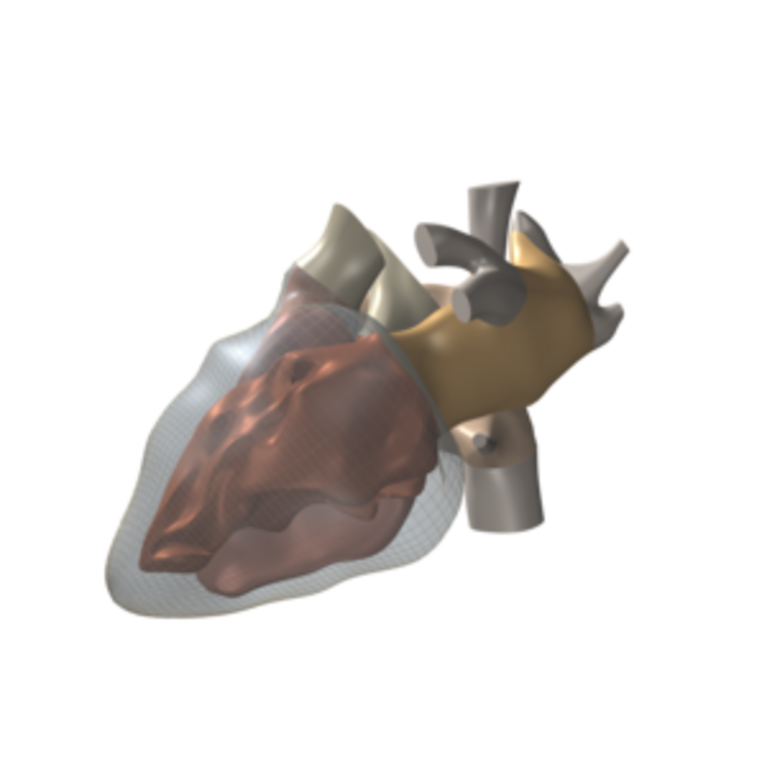
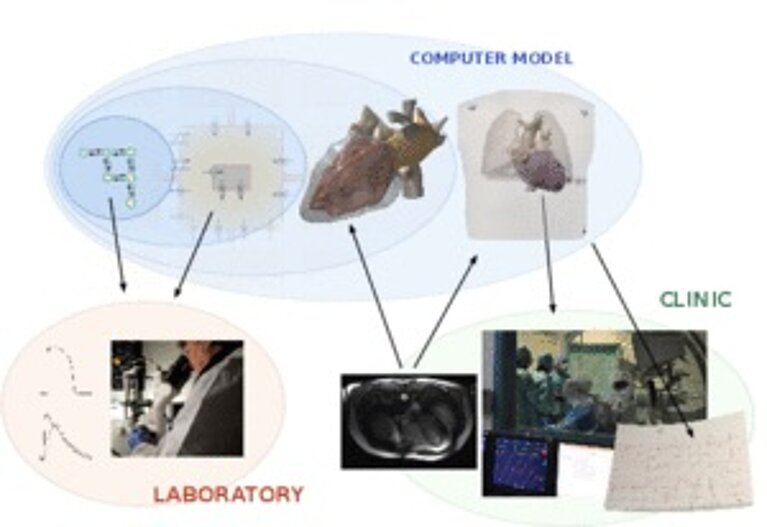
In order to be able to diagnose the causes, physicist Potse turned his attention to simulating the heart in every detail: the ion channels in the cell membrane that power the electric stimulus, its propagation through the heart muscle, and the resulting electric current flow in the human body. In other words, a supercomputer simulates the entire chemical-electrical process that activates the heart and is measured in the ECG.
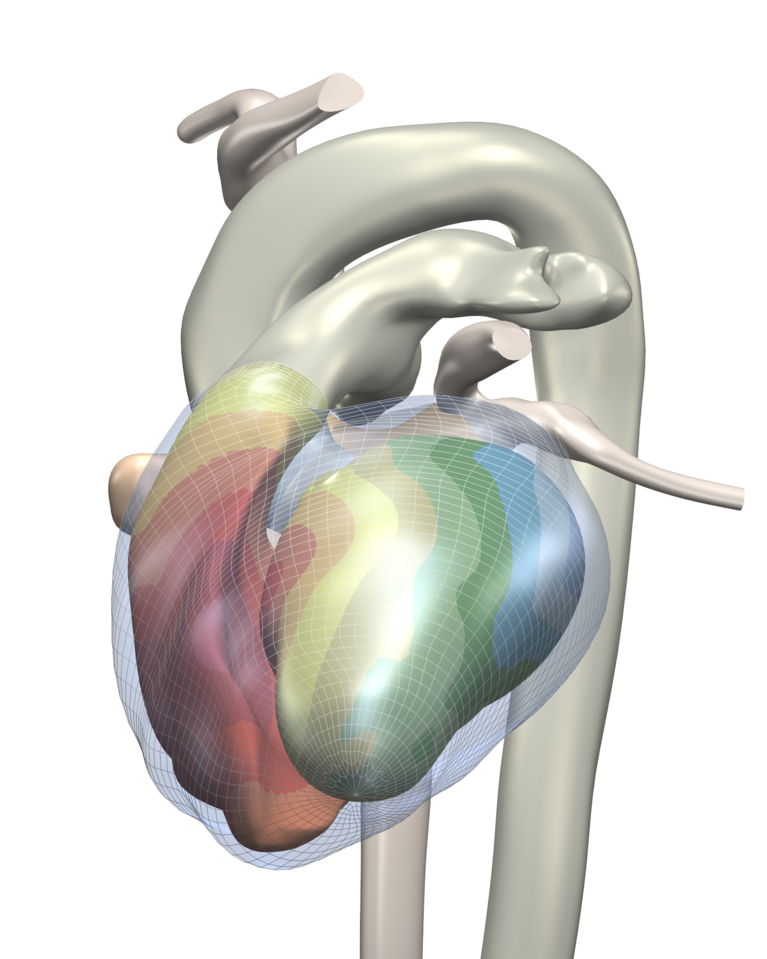
Highly detailed simulations like those conducted by Potse and his team are new in the nearly forty-year history of simulating electrocardiograms. What is simulated is the electrical current that spreads via the ion channels located in the cell membrane and its influence on other processes. “The ion channels activate a cell, for instance, so that a large number of sodium ions can flow in,” explains Potse. These can flow on into neighbouring cells through gap junctions and charge their cell membrane so that their potential becomes more positive. This in turn triggers the sodium flow at other points. The current flow causes a potential field in the whole body. The ECG measures these differences in potential.
The detailed simulations display interference up to cell level and thus render damage to the gap junctions of the ionic conductions between the cells visible. This enables the pathological consequences to be extrapolated. The method has thus been met with great interest among heart specialists. This resulted in a close international collaboration with scientists who conduct research and work in different areas of cardiology (see box) and the renowned Cardiocentro Ticino.
Collaboration with Cardiocentro Ticino
“The biggest challenge in the treatment of heart failure using electronic devices is to individualize the delivery of the therapy,” said Angelo Auricchio, Director of the clinical electrophysiology programme at Cardiocentro Ticino. Consequently, the researchers are currently producing computer models for twelve patients at the Cardiocentro Ticino to find out why a classic biventricular pacemaker does not help in thirty per cent of them. ECG data and magnetic resonance imaging is channelled into the models. The aim is to reproduce the signals that are measured in the patients accurately in the model. The scientists keep modifying their models until the results coincide with the values measured. For this purpose, they conduct 100 to 200 simulations for every patient. “This enables us to see which of our model hypotheses explain the patient’s pathological symptoms,” says Potse.
For the simulation on the CSCS supercomputer Monte Rosa, the researchers use a simplified grid that portrays the cardiac muscle. Cube corners of the elements that form the grid represent the individual grid points. Every one of the cube-shaped elements has an edge length of 0.2 millimetres. Depending on the patient, the cardiac muscle in the simulation is composed of about 100 million of these elements. In order to simulate the current flow and the potential field, the ion flow through the cell membrane is calculated for every grid point of the model based on differential equations.
Improving treatment methods
For Potse, the discovery that there is not always an LBBB despite ECG indications and additional damage could still be present is an important research breakthrough. Instead of a left bundle branch block, the scientist believes that the cause might also be muscle damage. This now needs to be clarified for individual cases with the models. Through the detailed diagnosis, the next step will be to seek a more suitable therapy. Depending on the cause, perhaps fitting the pacemaker electrodes in a different place might help.
The project is a joint initiative of Mark Potse, a guest professor at the Università della Svizzera italiana; Rolf Krause, Director of the Institute of Computational Science at the Università della Svizzera italiana; Angelo Auricchio, Director of the Clinical Electrophysiology Programme at the Cardiocentro Ticino; and scientists from the Politecnico Milano and Maastricht University. The international collaboration is funded by the “Iniziativa Ticino in Rete”