October 22, 2025 – by Santina Russo
Imagine an accident leaves you unable to walk. A simple fall or traffic crash can damage the spinal cord, and, in many cases, lead to near or full paralysis. Such an injury affects mobility, bodily functions, and independence, fundamentally changing the lives of those affected and their families. The challenges people with spinal cord injury face go beyond medical treatment. They are systemic: care is often fragmented and participation in society limited. In Switzerland, more than 8,000 people live with SCI, and around 240 new cases occur each year.
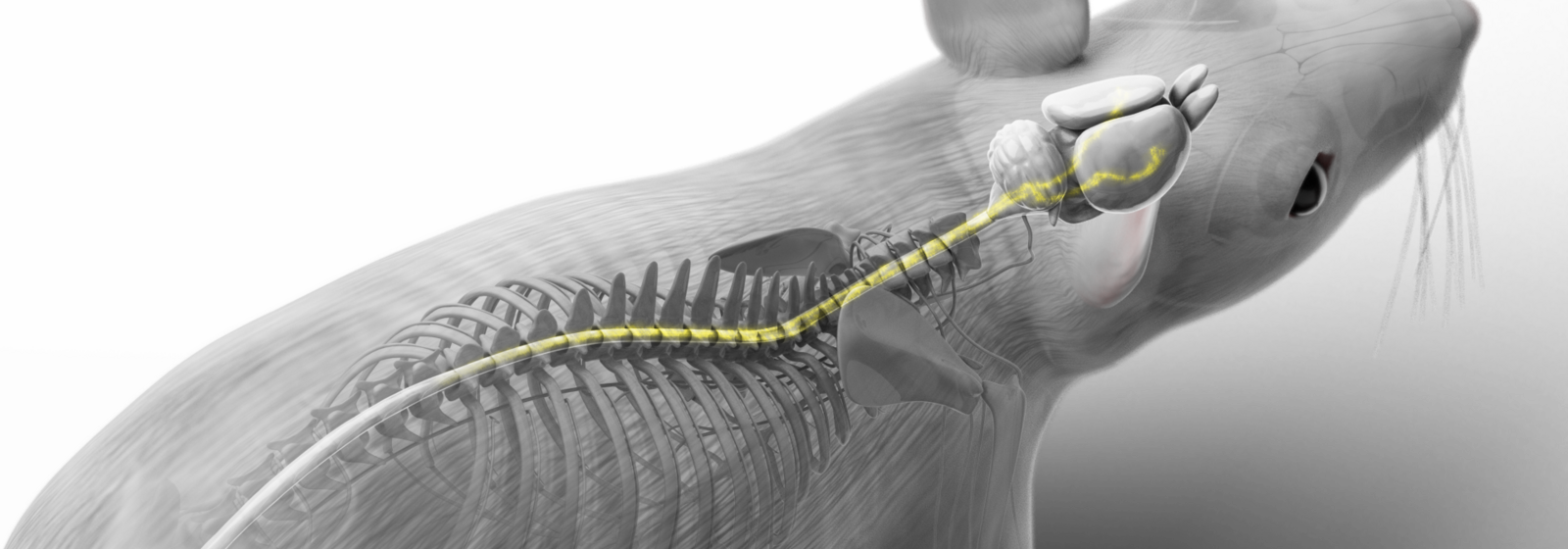
Now imagine that targeted brain stimulation could reverse the effects of a spinal cord injury (SCI) and help you walk again. That’s exactly what Quentin Barraud, a research scientist at EPFL and the Lausanne University Hospital (CHUV), and his colleagues are working on. Using samples from a mouse model, the team conducted extensive lab experiments and built a detailed virtual atlas of the human brain using CSCS’s supercomputing infrastructure. Their efforts culminated in a pilot clinical study, where they applied their insights to develop a deep brain stimulation therapy that allowed two participants to regain once lost walking ability.
A novel systematic inspection
This kind of recovery is possible because in most cases, even in severely paralyzed patients, spinal cord lesions are incomplete. Some nerve fibres remain intact, meaning that the brain can still access the neuronal circuits below the injury. In the past, empirical findings loosely suggested which brain regions might play a role in this connection, and possibly, for recovery.
Now, Barraud and his colleagues took this idea further, exploring the possibility of recovery in a systematic and comprehensive way: With CSCS’s supercomputing resources, they created a virtual brain atlas to assess which regions might aid recovery and how. To achieve this, they combined two brain-wide analyses related to SCI recovery.
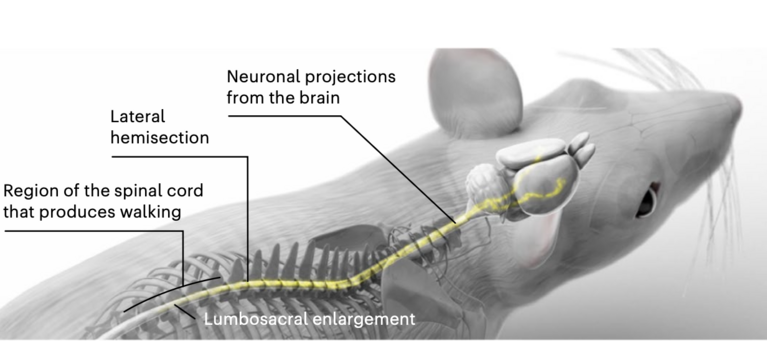
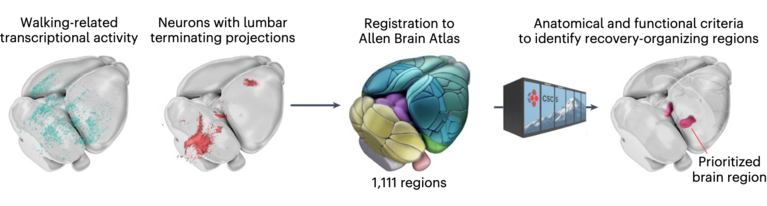
As a first step, the researchers identified neurons that become transcriptionally active after the injury. They used mice with a mild, incomplete SCI that allows the animals to naturally regain motor functions. To monitor this repair, the scientists used a biological marker that tags only neurons producing a specific protein called cFos, which is a trigger for a cascade of gene responses following the injury. “The idea was to compare the injury-triggered expression pattern in the mice’s brains with that of uninjured animals,” Barraud explained. For the second part of the brain atlas, the team mapped how neurons connect to body parts below the injury. By injecting a fluorescent tracer protein into the spinal cord below the lesion, they pinpointed neurons still linked to the circuits controlling the lower body.
Solving a picture puzzle of over 1,000 brain regions
To image the entire brain, the team used light sheet microscopy, a technique in which numerous layers of ultra-detailed 2D microscopic images are reconstructed into 3D model.
Each layer has a resolution of 2x2 micrometres, and the vertical resolution is 5 micrometres. This level of detail allowed the researchers to distinguish individual cell nuclei. However, to capture the whole brain, they had to image many smaller areas separately and then piece them together. “You can imagine single tiles of imaging data, which we then built into a virtual 3D sculpture of the brain,” Barraud explained. Processing and analysing such a massive, high-resolution dataset requires enormous memory and computing power—a task that would have been impossible without a supercomputer like CSCS’s then system “Piz Daint”.
The team also had to find a way to navigate within their imaging data. Simply put, they needed to figure out which tiles belonged next or on top of each other. For reference landmarks, they used of the open-access Brain Atlas from the Allen Institute in Seattle, a map of known brain regions. To align their new imaging data with the Allen Brain Atlas, the team leveraged an AI-enhanced computational pipeline called MIRACL, which they ran on the CSCS supercomputer.
Together, the two maps—one showing neuronal activity and other connectivity beyond the injury—formed a comprehensive brain atlas. Out of more than 1,100 brain regions, it highlighted those most likely involved in recovery after spinal cord injury.
A treatment for substantially improved walking
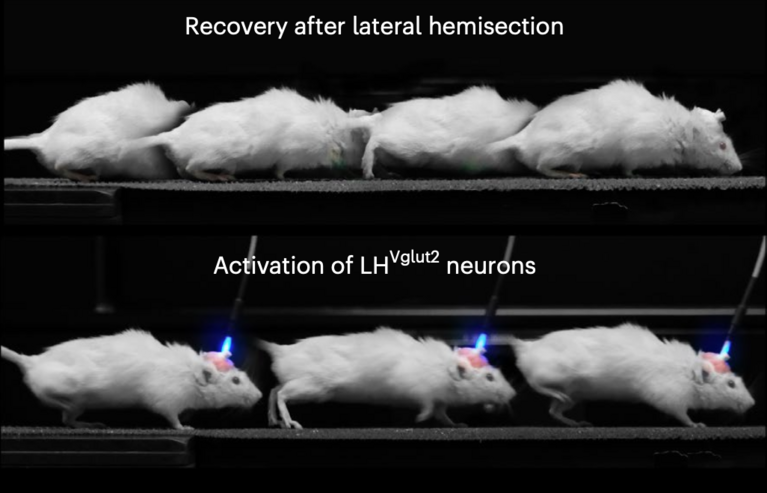
To the teams’ surprise, the results pointed to the lateral hypothalamus as the most relevant region for recovery. While several brain areas were active at different stages of injury and healing, only the lateral hypothalamus met all the key criteria: its neurons lost activity immediately after the SCI but reactivated during natural recovery. Similarly, these neurons lost connectivity to the paralyzed body parts right after injury but regained it as walking ability returned.
“The lateral hypothalamus is a highly specialized brain region primarily known for regulating arousal or feeding,” Barraud said. “That’s why we were surprised initially. But it had been linked to movement before, in old publications from the 1980s, which gave us confidence we were on the right path.” With these findings, the researchers proceeded to test what would happen if they specifically activated the neurons in that region in mice. They used two approaches: first, channelrhodopsin proteins, which can optically excite neurons; and second, electrical deep brain stimulation.
And indeed, when both stimulation methods targeted a certain type of neuron, the glutamatergic neurons that produce the neurotransmitter glutamate, the mice immediately regain walking ability, and their recovery continued to improve. “It was truly fascinating for everyone involved to see such an immediate improvement,” said Barraud.
Towards helping many patients
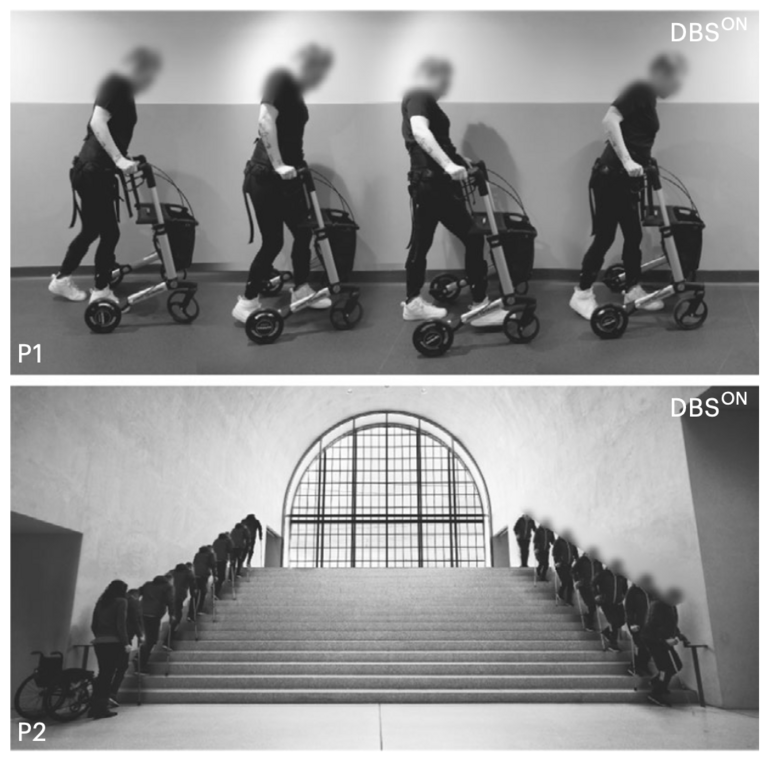
Finally, Barraud and his colleagues applied their findings in a pilot clinical study with two patients who had suffered incomplete spinal cord injuries. They used diffusion tensor imaging (DTI), a technique that indicates nerve pathways in the brain, to precisely localize the hypothalamic neurons linked to legs movement. The team then applied deep brain stimulation (DBS) to this specific area of the lateral hypothalamus to hopefully enhance the patients’ recovery. In this procedure, two thin, knitting-needle-shaped electrodes are implanted into the brain with the help of live imaging to find the exact target area. Their electrical impulses then activate the relevant neurons.
The result: There were no adverse side effects related to DBS, and both participants showed improved walking ability within days of treatment. The device was switched on for 30 minutes a day, and combined with three months of rehabilitation exercises it significantly supported recovery. “In our accompanying measurements, we saw that DBS enhanced the organization and plasticity of spinal cord circuits below the injury, leading to better recovery”, Barraud said.
One of the two patients improved so much that she relied less and less on her walking frame and, for the first time in years, felt confident enough to take walks outside independently. The second patient even managed to climb and descend stairs by his own thanks to the treatment.
Barraud and his colleagues are now conducting further studies to investigate and validate this approach, as well as follow-up studies with the same patients. One of their goals is to determine whether, once the electrodes are implanted, patients could administer the electrical impulses at home in their everyday lives—a step that could make this promising therapy accessible to many more people.
Reference:
N. Cho et al.: Hypothalamic deep brain stimulation augments walking after spinal cord injury. Nat Med30, 3676–3686 (2024). DOI: https://doi.org/10.1038/s41591-024-03306-x
Inline image credit: N. Cho et al, Nat Med (2024), DOI: https://doi.org/10.1038/s41591-024-03306-x