July 03, 2023 - by Santina Russo
It is the simplest and smallest of all negatively charged particles: the so-called aqueous electron. This free electron solvated in water occurs rather frequently. In ammonia, such a solvated electron is apparent by an intense blue colour. It is one of the most powerful reducing agents and is used, for instance, in electrode applications such as electrosynthesis, where chemical compounds are synthesized in electrochemical cells, or in electrodeposition, a process for producing a metal coating on a solid substrate.
Because of its apparent simplicity, the aqueous electron is the representation of one of the basic problems in quantum mechanics — but at the same time, it is extremely challenging to model. In fact, the aqueous electron has been studied for decades, on the one hand because of its role in radioactive chemistry, and on the other because it can serve as a deceptively simple system to compare predictions of quantum molecular dynamics simulations with experimental observations.
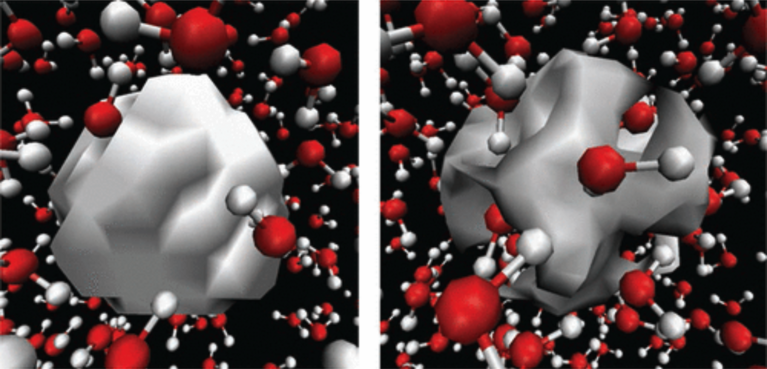
Despite the keen scientific interest, however, there are still uncertainties regarding the surrounding molecular structure. Undisputed is that the solvated electron’s charge is rather localized and not spread out over a large area or multitude of water molecules. But does it occupy a distinct cavity in between the water molecules? Or does it encompass a few water molecules, or maybe adopt a structure somewhere in between? Now, after performing simulations on the CSCS supercomputer “Piz Daint”, scientists in the group of Alfredo Pasquarello, professor of theoretical condensed-matter physics at EPFL in Lausanne, have helped to clarify this question.
To be or not to be in a cavity
Prior to the EPFL scientists’ work, most simulations of the aqueous electron and their corresponding experimental observations seemed to point to the cavity structure. However, there was one remaining property that no one had been able to explain with the cavity model before, namely a specific transition of the aqueous electron’s state dependent on temperature, which is visible as a red shift of the absorption peak in spectroscopic measurements. “In fact, only non-cavity models were able to reproduce this feature before”, said Pasquarello.
However, the cavity model was shown to explain many other experimental findings and is by many considered to be the likelier structure. That’s why Jinggang Lan, a postdoctoral researcher in Pasquarello’s group and first author of the respective paper, set off to find out if an extensive simulation using high-level quantum mechanics would explain this temperature dependent absorption peak and, in the best case, also reveal why it occurs.
Making the quantum world affordable
For their calculation, the team used ab-initio molecular dynamics simulations combined with machine learning (ML) techniques. “Molecular dynamics and machine learning methods work very well together,” stated Pasquarello, which is why the team employed ML methods to accelerate the simulations and extend the time scale without ballooning the cost in terms of computing resources. This enabled them to observe their system for longer and gain more detailed insight.
In the simulations, Jinggang Lan treated the solvent water molecules and aqueous electron with quantum mechanical methods, namely so-called hybrid functional molecular dynamics. “Simply put, this method enables simultaneous treatment of the aqueous electron and of all valence electrons of the water molecules — the ones in the atom’s outer shell — on an equal footing, allowing for a more realistic description of the aqueous electron,” Lan explained. “With this, the method is vastly improved compared to previous simulations, in which the water molecules were treated with classical methods and only the aqueous electron with quantum mechanics.”
The addition of the ML techniques then enabled Lan to accelerate the hybrid functional simulations. They typically comprise a time scale of around ten picoseconds (one picosecond is one trillionth of a second, equal to 10-12 seconds), and involve around 20,000 separate energy evaluation calculations, as Lan points out. With ML, he was then able to extend the observed time scale ten-fold to 100 picoseconds. It was this accelerated quantum-ML method that enabled the scientist to perform multiple simulations at different temperatures in the first place, which was necessary to study the temperature dependence of the absorption peak in question.
This video from Jinggang Lan’s and Alfredo Pasquarello’s and simulations shows how the aqueous electron’s charge density is distributed in the water solvent: It displaces the water molecules and occupies a rather secluded cavity. Some water molecules replace each other in their contact with the electron’s cavity, and the cavity is vibrating and changing, but it remains well formed over time. The video covers a time range of 15 picoseconds. (Video: Jinggang Lan)
The origin of the elusive peak
Within these extensive ab initio simulations, Lan and Pasquarello could indeed observe that the aqueous electron’s charge density forms a distinct cavity within the solvent, and the hydrogens of the water molecules face the electron’s cavity.
In addition, by comparing simulations of the system at various temperatures within a range from 300 K to 600 K, the scientists discovered the origin of the temperature dependent shift of the spectral absorption peak. By analysing their numerous simulation runs, the scientists found that the observed temperature dependence is related to the aqueous electron’s cavity size: the higher the temperature, the larger the localization radius of the aqueous electron, meaning the cavity in which it resides. This change in the electron’s extension, in turn, presents as the observed absorption peak shift in spectra.
“The dependence we found is actually rather straightforward,” stressed Pasquarello. “Now that we have deciphered the correlation, we are able to describe it by a fairly simple model.” Also, by observing the aqueous electron forming a cavity structure — and by explaining the absorption peak feature in accordance with this structure — the EPFL scientists provided the strongest confirmation of the cavity model to date.
Image above: Alkali metals such as lithium, sodium, or potassium dissolve readily in liquid ammonia to form, among other things, solvated electrons. Their presence is visible by the intense blue colour. (Image: Klein research group, Ch. Joest)
References:
J. Lan, V.V. Rybkin and A. Pasquarello: Temperature dependent properties of the aqueous electron, Angew. Chem. Int. Ed.61 (2022), e202209398, DOI http://doi.org/10.1002/anie.202209398