November 26, 2019 - by Simone Ulmer
The intact human immune system works as precise as a clockwork: it repels infections and cancer cells without harming the otherwise healthy organism. But if the immune system is not intact, those affected are usually dependent on medical help. Many patients with genetically-caused primary immunodeficiency disorders (PIDs) suffer from a lack of antibodies (primary antibody deficiency), which are normally produced in so-called B cells. As a result, they are susceptible to infections, but also to autoimmune and autoinflammatory diseases. Recently, an international team of researchers has succeeded in deciphering an essential molecular process in the B cells of a subgroup of affected patients – thus enabling tailored and more efficient treatment.
Increased cell respiration due to mutation
The researchers, led by Christoph Hess and Mike Recher, both professors at the Department of Biomedicine of the University of Basel and the University Hospital of Basel as well as at the University of Cambridge, have taken a close look at the cellular metabolism of B cells in PID. They found that in these patients, the power plants of the cell – the so-called mitochondria – exhibit increased cellular respiration. In the affected patients, researchers also found a mutation in the germ line of the protein SDHA, which is a key component of the respiratory chain of the cell.
Co-author Olivier Bignucolo from the University of Lausanne used the Swiss National Supercomputing Centre (CSCS) supercomputer "Piz Daint" to visualize the molecular dynamics of SDHA in these cells. Bignucolo is first assistant at the Department of Pharmacology and Toxicology and a computational structural biologist specialized in molecular dynamic simulations. Using "Piz Daint" and a special software, Bignucolo attempted to clarify the cause of the increased cellular respiration in the B cells from these patients. "Molecular dynamic simulations complement conventional imaging techniques perfectly, as they enable the analysis of the biological system over time at the atomic level," Bignucolo notes. “The behaviour of ions, water and any ligand are assessed together with the proteins of interest.” Using simulations, the researchers were able to describe a modified network of atomic interactions caused by SDHA mutations.
Disturbed respiratory chain promotes inflammatory reactions
The simulations showed that the mutation of the protein SDHA enhances its interaction with SDHB, thus augmenting the activity of the respiratory chain and driving accumulation of a certain salt (fumarate), which in turn activates a signalling cascade leading to the production of so-called cytokines which ultimately cause inflammatory reactions. On the basis of these results, the researchers were able to treat one patient with an antibody to specifically block a key inflammation mediator (cytokine) triggered by the increased interaction of the two proteins SDHA and SDHB. This treatment reduced systematic inflammation and the patient's condition improved, according to the scientists. "The decoding of such processes not only clarifies basic biological mechanisms, but also enables more targeted treatments or the production of drugs with fewer side effects," emphasises the physician Christoph Hess.
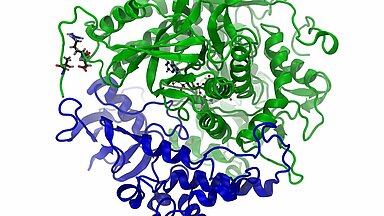
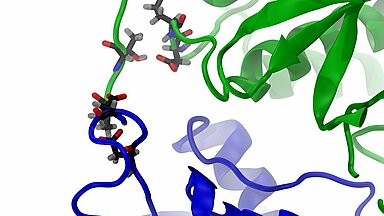
Enhanced SDHA/SDHB interactions in the mutant
The video highlights the interaction between SDHB (blue) and SDHA (green): on the right hand the wild type, left the mutated SDHA. The coloured spheres representing the interacting atoms: carbon, oxygen and nitrogen. The interactions between the mutated variant of SDHA and SDHB left lasts significantly longer.
Reference:
Burgener, A et al. SDHA gain-of-function engages inflammatory mitochondrial retrograde signaling via KEAP1–Nrf2. Nature Immunology 20, 1311–1321 (2019) doi:10.1038/s41590-019-0482-2